Stanford scientists figure out a way to get hydrogen out of seawater. Does this matter?
Every time the words “hydrogen fuel” come up, I want to yell in bold uppercase that if it is made through electrolysis, “HYDROGEN ISN’T A FUEL, IT’S A BATTERY!” And come up it has, in Fast Company, where Adele Peters writes Scientists just found a new way to make fuel from seawater.
She describes a new improvement where hydrogen can now be electrolysed from seawater without the anodes dissolving because of the salt. Stanford researchers figured out how to coat the anode to keep it from corroding, according to the press release:
The researchers discovered that if they coated the anode with layers that were rich in negative charges, the layers repelled chloride and slowed down the decay of the underlying metal….Without the negatively charged coating, the anode only works for around 12 hours in seawater, according to Michael Kenney, a graduate student in the Dai lab and co-lead author on the paper. “The whole electrode falls apart into a crumble,” Kenney said. “But with this layer, it is able to go more than a thousand hours.”
Peters at Fast Company writes:
The fuel could theoretically be widely used in transportation, from cars to planes… Hydrogen fuel cells could also store electricity from power plants or store energy in houses.
This is what makes me crazy. Ok, it is true that we have a lot of saltwater around. But it doesn’t change the physics or chemistry of how much energy it takes to split water into hydrogen and oxygen. It’s a lot of energy; let’s pick an example and look at the thermodynamics of running a Toyota Mirai on saltwater hydrogen (and I welcome criticism of my math here).
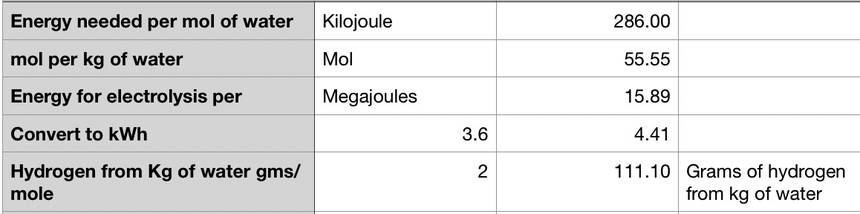 electrolyzing water takes energy/ spreadsheet by Lloyd Alter/CC BY 2.0
electrolyzing water takes energy/ spreadsheet by Lloyd Alter/CC BY 2.0

 ©
©